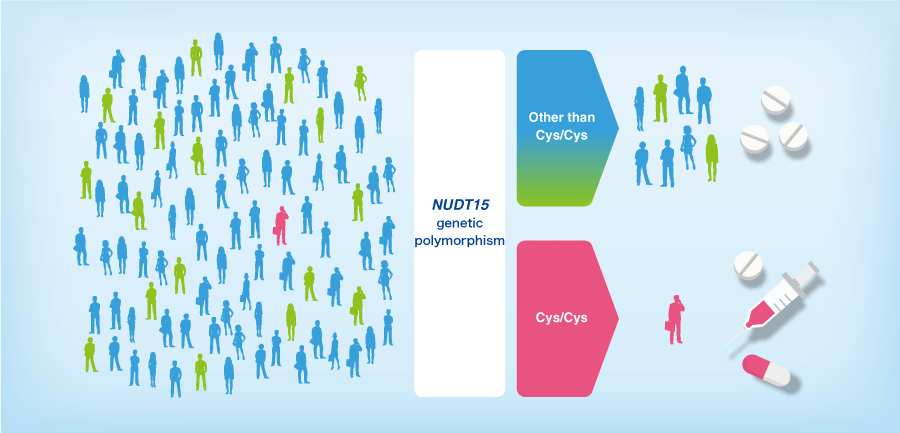
The thiopurine drugs are widely used to treat. However, the incidence of adverse reactions is high, particularly in Asia.
NUDT15 genotyping is a good candidate for predicting thiopurine toxicity in East Asian populations. (Kakuta et al. 2018)
Nudix hydrolase 15 (NUDT15) genetic polymorphism kit
*Thiopurine drugs are used to treat patients such as SLE, IBD and Behcet's disease.
**PMDA: Pharmaceuticals and Medical Devices Agency
Request documents
Contact Us
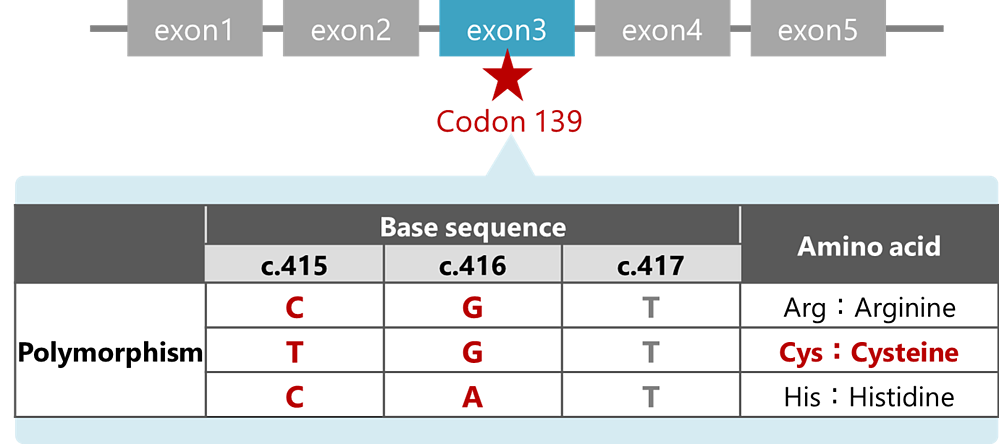
is a reagent for assessing three types of alleles by detecting genetic polymorphism at codon 139 of the NUDT15 gene. The kit adopts the real-time PCR method and uses genome DNAs extracted from whole blood as specimens. The kit allows to predict patients with a high risk of serious adverse events from thiopurine drugs and provides support in selecting treatment options for those with reduced NUDT15 activities.
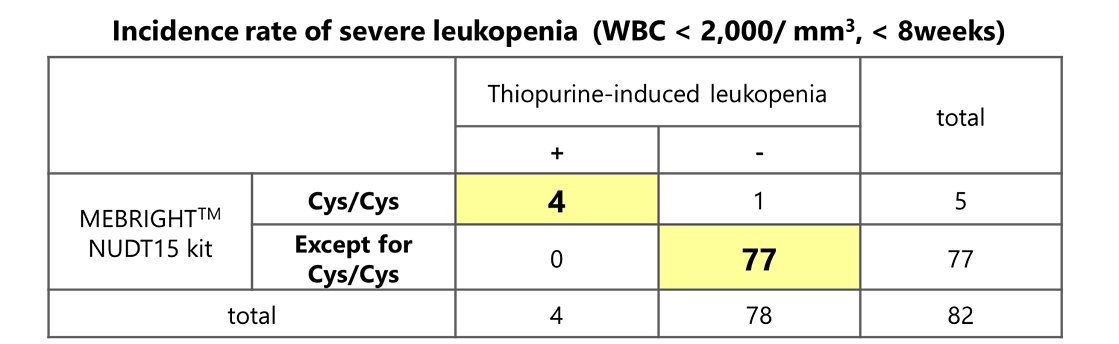
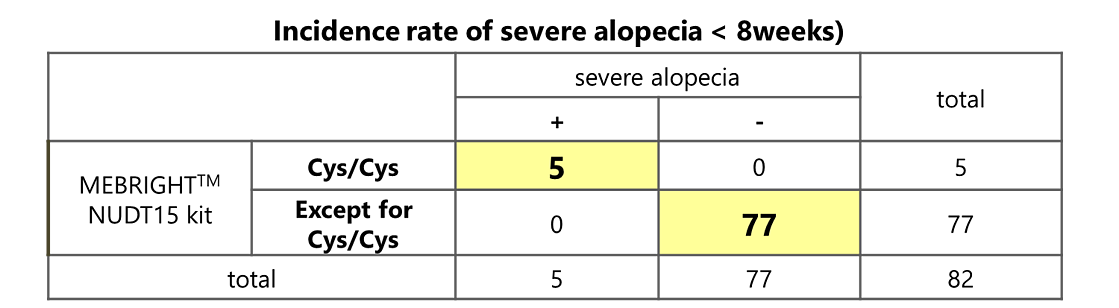
Detection results of this product and the incidence rate of side effects due to thiopurine-type immunoregulatory agents.
Kakuta et al. (2018). "Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping." Journal of Gastroenterology 53(2): 172-180.
MEBRIGHT™ is a registered trademark of Medical & Biological Laboratories Co., Ltd.
Products may not be approved for sale as in vitro diagnostics in all countries. Please contact us for further details.
Request documents